Business Type:Others
Country/Region:China
Ddu Verified
HOT Rank


Nanjing Synthgene Medical Technology Co., Ltd
Nanjing Synthgene Medical Technology Co., Ltd. is a high-tech enterprise focusing on the development, production of in vitro diagnostic (IVD) materials and POCT diagnostic reagents. The company has
Business Type:Others
Country/Region:China
Ddu Verified
HOT Rank

 【Product name】 Malaria pf/pv Antigen Rapid Test Kit (Colloidal gold method)
【Product name】 Malaria pf/pv Antigen Rapid Test Kit (Colloidal gold method)
【Packaging specifification】 1 test/bag, 1/5/10/25/50 test(s)/kit
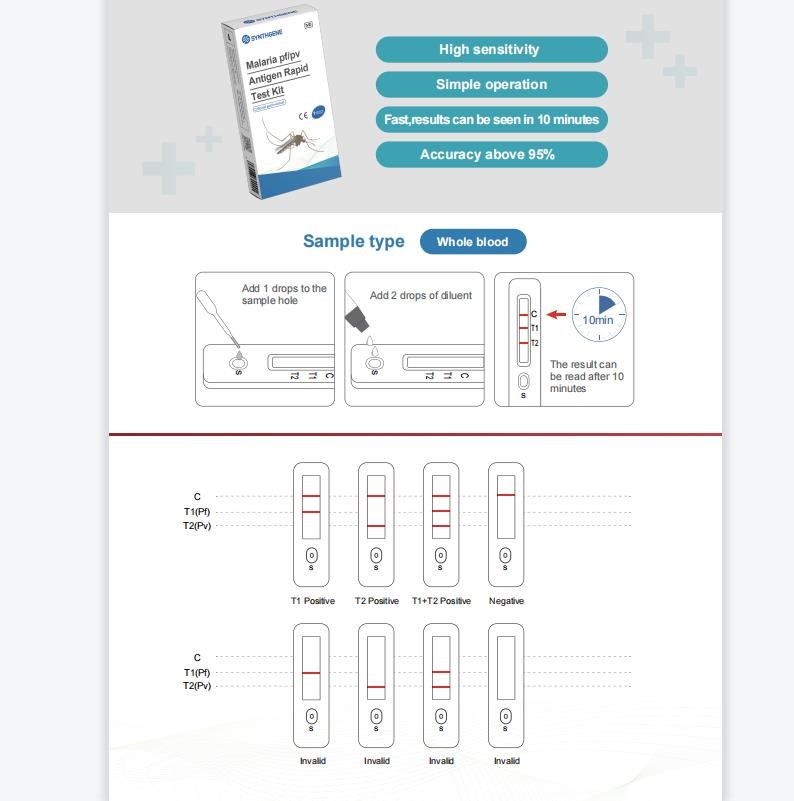
【Intended use】 This product is used for in vitro qualitative detection of Plasmodium falciparum antigen (P.f.) and Plasmodium vivax antigen (P.v.) in human whole blood samples. This product is suitable for qualitative detection of Plasmodium falciparum antigens and Plasmodium vivax antigens in whole blood samples of people with malaria symptoms. Plasmodium vivax makes a differential diagnosis, this product does not require any instrument, the whole detection process is simple, and has the characteristics of rapidity, accuracy and high sensitivity.
【Testing principle】 This product is a rapid diagnostic reagent developed based on immunocolloidal gold technology and immunochromatography technology. Plasmodium falciparum antigen and Plasmodium vivax antigen in blood samples were qualitatively determined by double-antibody sandwich method, and the detection lines T2, T1 and control lines on nitrocellulose membrane were coated with P. vivax antibody, P. Plasmodium antibodies and goat anti-chicken IgY. For detection, the sample is applied to the sample pad, and the sample is chromatographed under capillary phenomenon, moving forward along the paper strip. If the sample contains P. vivax antigen, the labeled Au-P. vivax antibody complex is combined with the detection line to form "Au-P. vivax antibody-P. vivax Ag--P. vivax antibody", if the sample contains Plasmodium falciparum antigen, the labeled Au-P. antibody "sandwich" aggregates and develops color, resulting in a red band at the detection line (T1 or T2). The free colloidal gold-labeled chicken IgY reacts with goat anti-chicken antibody at the control line and a red band appears; no matter whether there is malaria parasite antigen in the tested sample, the complex will continue to be chromatographed up to the quality control line (C ), a purple-red band appeared in the reaction. The red band presented by the quality control line (C) is the standard for judging whether the chromatography process is normal or not, and also serves as the internal control standard for the reagent.

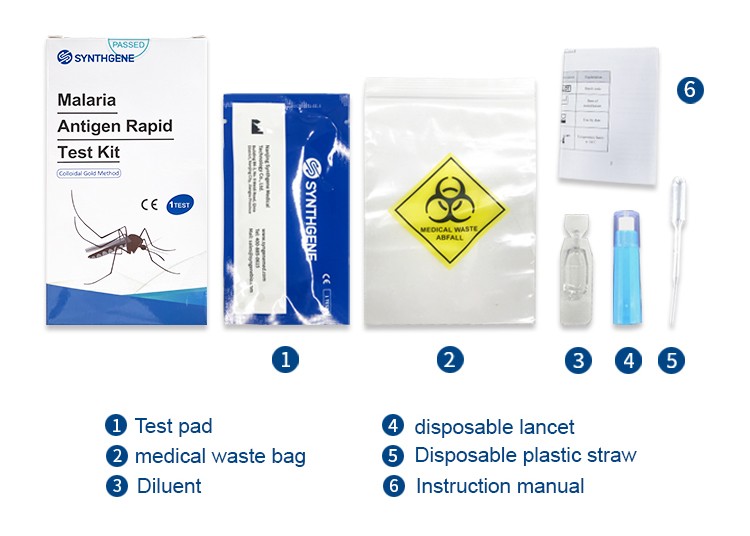
 【Main components】
【Main components】
1.Test pad (1 piece/bag, 1/5/10/25/50 piece(s)/kit)
2.Disposable plastic straw(1 piece/bag, 1/5/10/25/50 piece(s)/kit)
3.Sample diluent (1 piece/bag, 1/5/10/25/50 pieces(s)/kit)
4.Instruction manual (1copy/bag, 1copy/kit)
Note: The components in the kits of different batch numbers are not interchangeable.