Business Type:Others
Country/Region:China
Ddu Verified
HOT Rank


Nanjing Synthgene Medical Technology Co., Ltd
Nanjing Synthgene Medical Technology Co., Ltd. is a high-tech enterprise focusing on the development, production of in vitro diagnostic (IVD) materials and POCT diagnostic reagents. The company has
Business Type:Others
Country/Region:China
Ddu Verified
HOT Rank

【Product Name】 SARS-COV-2 Virus Neutralization Test Kit (Colloidal gold method)
【Packaging specification】 1/5/25/50 test(s)/kit
【Intended use】 This product is used for in vitro qualitative detection of novel coronavirus (SARS-COV-2) neutralizing antibodies in human serum/plasma/whole blood/fingertip blood. The 2019-nCoV neutralizing antibody is a protective antibody produced by the human body after being vaccinated with the novel coronavirus vaccine or infected with the novel coronavirus. Neutralizing antibodies have the function of recognizing the surface S-RBD protein of the novel coronavirus, blocking the binding of S-RBD to the cell surface specific receptor (ACE2), and exerting an antiviral effect. Whether neutralizing antibodies are produced and the amount or titer produced is an important indicator of the immune protection effect after vaccination with the novel coronavirus vaccine, and it is also an important basis for vaccine evaluation and quality control. One of the important indicators of its cure evaluation, it is helpful for the screening of novel coronavirus vaccinated population and the judgment of the effect after vaccination.
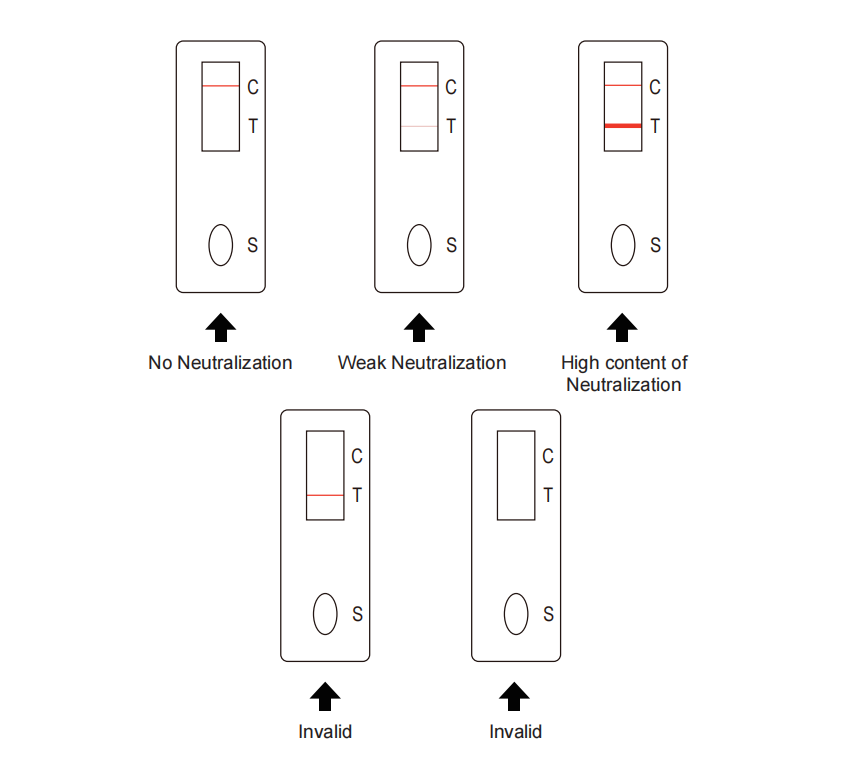
【Testing principle】 This test kit detects the neutralizing antibody of 2019-nCoV by S-RBD double antigen sandwich method. Take the sample from vaccinated people and drop it on the sampling area of the test pad, add two drops of diluent, the blood sample will be chromatographed forward under the capillary action. After passing the sample pad containing ACE2 and the colloidal gold binding pad labeled with RBD protein, the sample will enter the nitrocellulose membrane area. If the sample does not contain neutralizing antibodies, the ACE2 in the sample pad will bind to the RBD protein in the binding pad, and the detection line (T line) will not develop color. If the sample contains neutralizing antibodies, it will block the binding of ACE2 in the sample pad and the RBD on the colloidal gold binding pad, and the colloidal gold-labeled S-RBD will be produced on the detection line (T line), and the antibody in the serum will conjugate with the RBD coated on the detection line (T line), which manifest as color development of the detection line (T line). The antibody content in the serum is proportional to the detection line color intensity. After the reaction, the color development can be directly observed with the naked eye.

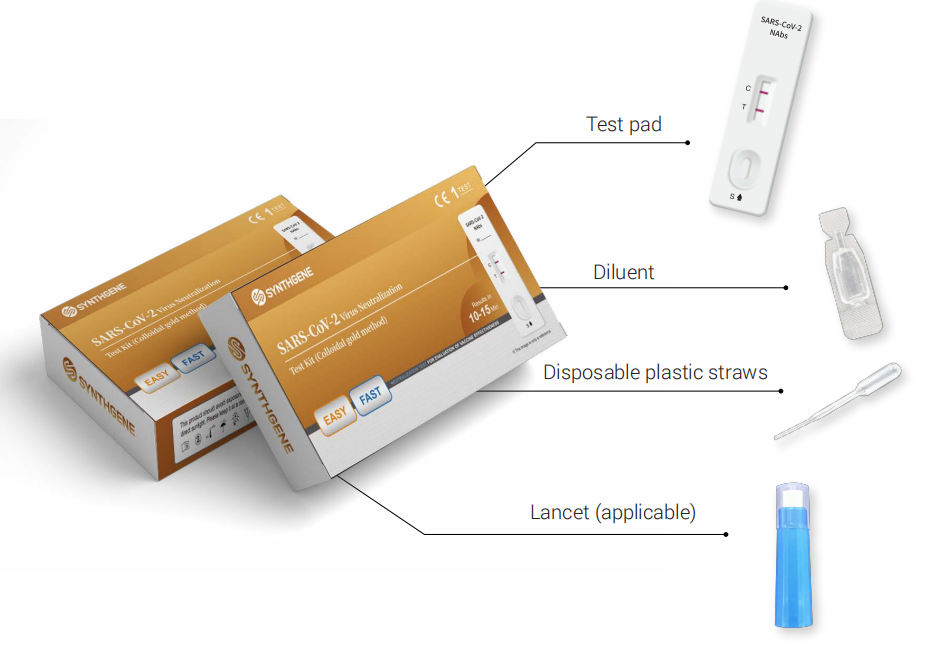
【Main components】
1. Test pad, individually packed in aluminum foil bag(1/5/25/50 piece(s)/kit )
2. Sample diluent(1/5/25/50 piece(s)/kit)
3. Disposable plastic straw (1/5/25/50 piece(s)/kit)
4. Instruction manual (1 copy/kit)